The difference in A1C effect observed in the trial may not necessarily reflect the effect that is observed in the care setting where alternative insulin glargine U-100 dosage can be used.
DUAL VII demonstrated A1C control and additional clinical benefits for adults with type 2 diabetes taking Xultophy® 100/3.6


DUAL VII demonstrated A1C control and additional clinical benefits for adults with type 2 diabetes taking Xultophy® 100/3.6
PRIMARY ENDPOINT OF NON-INFERIORITY ACHIEVED IN DUAL VII, A 26-WEEK, OPEN-LABEL TRIAL VS BASAL-BOLUS THERAPY, BOTH PLUS MET
Comparable A1C control vs basal-bolus therapy1
aEstimated treatment difference (ETD) (95% CI): –0.02 (–0.16; 0.12). P<0.0001 confirmed non-inferiority.

Study design
- Duration
- 26 weeks
- Patient population
- 506 adults with type 2 diabetes inadequately controlled on insulin glargine U-100 (<50 units) + MET
- Primary endpoint
- Change in A1C
- Select secondary endpoints
- Insulin dose, episodes of hypoglycemia, change in body weight, and proportion of patients who achieved A1C <7%, had no hypoglycemia in the last 12 weeks of treatment, and/or had no weight gain
Summary: Once-daily Xultophy® 100/3.6 vs basal-bolus therapy

insulin glargine U-100
insulin glargine U-100 + insulin aspart
insulin glargine U-100 + insulin aspart
PRIMARY ENDPOINT
Mean A1C reduction1,e
-1.5%
-1.5%
SECONDARY ENDPOINTS
Severe or BG-confirmed symptomatic hypoglycemia reported1,f
1.1 events/PYE
8.2 events/PYE
Weight change1
7.9 LB DIFFERENCE
-2.0 lb
+5.7 lb
Average insulin dose1,g
40 units
84 units
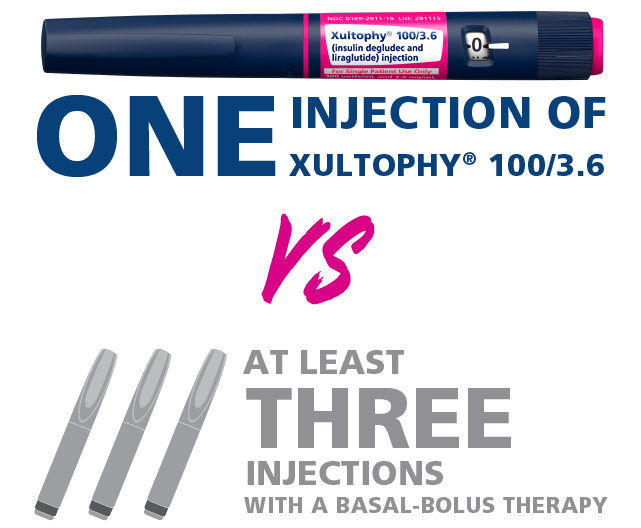
Number of injections1
1 injection
At least 3 injections
Weight gain can occur with insulin-containing products, including Xultophy® 100/3.6, and has been attributed to the anabolic effects of insulin.2
eThe difference in A1C effect observed in the trial may not necessarily reflect the effect that may be observed in the care setting where alternative insulin glargine and insulin aspart dosage can be used.
fSevere or BG-confirmed symptomatic hypoglycemia: an event requiring assistance from another person to actively administer carbohydrate, glucagon, or other resuscitative actions or BG-confirmed by a plasma glucose value (<56 mg/dL) with symptoms consistent with hypoglycemia.
gAverage end-of-trial dose was 40 units of Xultophy® 100/3.6 vs 52 units basal + 32 units bolus.
BG=blood glucose; CI=confidence index; ERR=estimated rate ratio; MET=metformin; PYE=patient-year of exposure.